How Can I Get a Lethal Dose of Fentanyl
Introduction
Opioid overdose deaths continue to increase in the United states, killing more than 42,000 people in 2022. The opioids detected in these cases, in increasing guild, were methadone, natural and semi-synthetic opioids (due east.thousand., oxycodone, hydrocodone), heroin and synthetic opioids (e.g., fentanyl, fentanyl-analogs). Synthetic opioids (excluding methadone) and heroin deaths specifically experienced a sharp increase from 2015 to 2022 (20 and 100%, respectively) (Seth et al., 2018). Fentanyl and its derivatives have been increasingly nowadays equally adulterants mainly in heroin, but too in other drugs such as cocaine and constructed cannabinoids (Coopman and Cordonnier, 2017; Armenian et al., 2018), due to their ease of manufacturing and readily available precursors shipped from Red china (Armenian et al., 2018). In addition to being nowadays in other drugs supply, fentanyl analogs have been also marketed every bit "research chemicals" and tin easily exist acquired over the net. Due to their high say-so and the increased utilize of heroin equally an initiating opioid of corruption (8.7% in 2005 vs. 33.3% users in 2015) (Cicero et al., 2017; O'Donnell et al., 2017), the number of opioid-related deaths have drastically increased in the recent years. Given that opioid novices have limited tolerance to opioids, a slight imprecision in dosing inherent in heroin employ and/or the presence of potent fentanyl and analogs, can be fatal.
Fentanyl, its analogs (e.g., acetyl fentanyl, 3-methylfentanyl, alphamethylfentanyl, furanyl fentanyl) and the new generation synthetic opioids (e.one thousand., AH-7921, U-47700, MT-45) accept a chemical cadre structure totally unlike from morphine, a naturally occurring opioid from Papaver somniferum and reference chemical compound of the opioids group; but all of them human action on the opioid receptor (mu-receptor) reducing the intensity of hurting and showing a high habit potential. These opioid receptor agonists also induce dose-dependent respiratory depression (Pattinson, 2008), which is the main reason for their life-threatening risk (Ujváry et al., 2017). Fentanyl is approximately 200 times more strong than morphine, and the potencies of its analogs are variable, from 7 times more potent than morphine for butyrfentanyl and furanyl fentanyl, to more than than 4,000 and 10,000 times for sufentanil and carfentanil, respectively (UNODC, 2017). The new generation opioids AH-7921 and MT-45 show similar authority to morphine (Brittain et al., 1977; EMCDDA, 2015), and U-47700 well-nigh seven.five times more potent (Cheney et al., 1985).
Synthetic opioids are widely regulated by the U.s.a. Controlled Substances Act of 1970 (CSA) in order to control their use and distribution. As new compounds arise and threaten public condom, compounds tin can exist emergency scheduled by the DEA to wearisome production and utilize of these harmful substances and aid in prosecution of drug diverters for a temporary period until the formal procedures take gone through (US Drug Enforcement Assistants, 2017). Substances are classified into schedules in the CSA based on their safety, medicinal employ and potential for abuse. A Schedule I substance is classified equally having no currently accepted medical utilize and a high abuse potential. Examples of constructed opioids in Schedule I include furanyl fentanyl, U-47700, acetyl fentanyl and iii-methyl fentanyl. Schedule 2 classified opioids have a high potential for corruption just take electric current medicinal uses like fentanyl which is used as an coldhearted and analgesic, every bit well as carfentanil, remifentanil and sufentanil (US Drug Enforcement Administration, 2017). Most recently, the DEA issued a temporary scheduling club for all fentanyl –related substances (to include all analog modifications) in Feb of 2018, which cover all substances that were non already classified into Schedule I of the CSA in an aggressive effort to regulate the manufacture and subsequent trafficking of new synthetic opioids into the United States (Drug Enforcement Administration, 2018).
The expansion of these new constructed opioids constitutes an of import challenge in forensic toxicology. First of all, most of these substances are not detected in the routine screening and confirmation methods in the laboratory. Also, due to the low doses employed of these highly potent drugs, the concentrations expected in the biological samples are in the low ng to pg/mL or ng to pg/g range, requiring extremely sensitive methods of analysis. Recently, Marchei et al. (2018) and Liu et al. (2018) reviewed the currently available screening and confirmation methods of new constructed opioids in biological and non-biological samples. As indicated by Marchei et al. (2018), gas chromatography combined with mass spectrometry (GC-MS) and more frequently liquid chromatography tandem mass spectrometry (LC-MSMS) are the most common techniques due to their sensitivity and specificity. Still, given the connected development of new derivatives, the major disadvantage of these target techniques, which utilise quadrupole mass spectrometers, is that are limited by the reference standards bachelor. High resolution mass spectrometry (time-of-flying, orbitrap) offers potential advantages to place unknown compounds without the availability of a reference standard, but this technology is not readily available in most forensic laboratories (Marchei et al., 2018).
Regarding biological samples, near of these methods take been developed in blood or urine, and the target analytes are the parent compounds and rarely the metabolites (Marchei et al., 2018). In postmortem toxicology, other biological specimens such as vitreous humor, liver and brain are commonly analyzed. Unfortunately, fully validated methods for the conclusion of synthetic opioids in these specimens are lacking in the literature. This is in role due to the abiding changes in illicit synthetic opioids existence identified and laboratories beingness unable to justify the extensive time and toll associated with fully validating a method for a drug that may only exist present in cases for a brusque fourth dimension. Analytical methods in forensic toxicology are normally validated in the respective biological sample following the guidelines published by the Scientific Working Group in Forensic Toxicology (SWGTOX) (Scientific Working Group for Forensic Toxicology, 2013) to guarantee the analytical quality of the measured concentrations. The analysis of metabolites in the different biological matrices may improve the interpretation of the results, extending the detection window and indicating if it was an acute or a delayed-death evaluating the metabolite-to-parent ratios. Recent publications about the identification of new metabolites of the synthetic opioids are bachelor (Wohlfarth et al., 2022; Steuer et al., 2017; Watanabe et al., 2017; Krotulski et al., 2018a); however, its application to authentic samples is nonetheless deficient (Poklis et al., 2015; Staeheli et al., 2022; Martucci et al., 2017; Allibe et al., 2018).
Besides the analytical challenges associated with constructed opioids, due to the scarcity of available postmortem data, the interpretation of the results is extremely difficult. Conducting postmortem toxicology interpretation provides a number of very pregnant challenges to the forensic toxicologist. The range of postmortem specimens (blood, urine, vitreous humor, tissues, pilus), the lack of reference databases, the presence of other substances (due east.1000., benzodiazepines, booze), opioid tolerance, and postmortem phenomena (postmortem redistribution and drug instability) complicates the interpretation of the analytical findings. Pichini et al. (2018) and Zawilska (2017) discussed non-fatal and lethal intoxications involving the new synthetic opioids, and Drummer (2018) focused his review on fatalities due to these compounds.
The nowadays review is focused on fentanyl derivatives and new generation opioids due to the express noesis concerning these substances and their high prevalence in opioid-overdose related cases. This work complements the previously published literature reviewing the current cognition of postmortem toxicology of synthetic opioids and the chemical and pharmacological factors that may bear on drug concentrations in the unlike matrices and therefore, their interpretation in postmortem samples. These factors include key chemical properties, essential pharmacokinetics parameters, postmortem redistribution and stability data in postmortem samples. All of these data are critically compared to postmortem data of natural opioids (morphine), semi-constructed (oxycodone, hydrocodone, hydromorphone, and oxymorphone), and constructed opioids (methadone and buprenorphine). The interpretation of drug intoxication in death investigation is based on the available published literature. This review serves to facilitate the evaluation of cases where constructed opioids may exist implicated in a fatality through the review of peer reviewed published case reports and inquiry articles.
Methods
PubMed, Scopus and Google Scholar were searched for appropriate articles. Forensic case-reports and enquiry articles of natural, semi-synthetic and constructed opioids were reviewed upward to May 2018. All manufactures were manually reviewed for content and references in each manuscript were further queried. Included manufactures were limited to peer-reviewed journals indexed by the Establish for Scientific Information (ISI) and published in English. Chemic properties were retrieved from the public databases PubChem (https://pubchem.ncbi.nlm.nih.gov/) and DrugBank (https://world wide web.drugbank.ca/drugs).
Chemic and Pharmacological Properties
The chemical construction of the diverse synthetic opioids, including fentanyl and analogs, differs significally from the chemical structure of morphine and semi-synthetic opioids (e.thou., oxycodone, hydrocodone, buprenorphine). Effigy 1 summarizes the chemic structure of selected classic opioids. Fentanyl is a piperidinyl derivative with moieties on the nitrogen and the 4-position (Figure 2). The different fentanyl derivatives prove substitutions on the propionyl moiety (e.thou., acetylfentanyl, acrylfentanyl, butyrfentanyl, furanyl fentanyl), phenethyl moiety (due east.g., ohmefentanyl), Northward-phenyl band (e.g., ocfentanil, 4-methoxy-butyrylfentanyl) and/or at the 4-piperidinyl-position (e.g., carfentanil). The chemic structures of the new generation constructed opioids (AH-7921, U-47700, MT-45) are different from fentanyl. Figure three shows 20 fentanyl derivatives and 3 new generation synthetic opioids not related to fentanyl. Due to the shut chemical structure among fentanyl derivatives, some compounds, such as cyclopropyl fentanyl and crotonyl fentanyl, take exactly the same molecular formula, and therefore, the same molecular weight. As a result of this, special attending has to be paid in the evolution of the belittling methods for the decision of these compounds, and a complete chromatographic separation is required to guarantee their right identification past gas or liquid chromatography coupled to mass spectrometry (GC-MS, LC-MSMS).
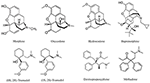
Figure 1. Chemical structures of selected classic opioids.
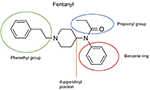
Figure 2. Chemic structure of fentanyl.
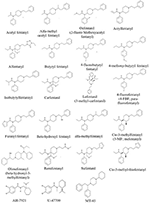
Figure 3. Chemical structures of xx fentanyl derivatives and iii new generation opioids non related to fentanyl.
Chemically, opioids are predominantly basic drugs with pKa ranging from 7.5 to 10.9. The chemic parameter log P, the decimal logarithm of the partition coefficient Kp, is a useful indication of the lipophilicity of a chemical compound. In the instance of opioids, log P range is wide, from 0.viii (oxymorphone) to v (methadone). Morphine and related compounds bear witness the lowest log P values (0.viii–two). Fentanyl and analogs show a log P between 1.5 and 4.iii. The high lipophilicity of fentanyl and its analogs enables rapid diffusion through membranes, including the blood-encephalon barrier. Also, this lipophilicity forth with their basic characteristics brand these grouping of drugs candidates to undergo postmortem redistribution. Table 1 summarizes the molecular weight, pKa and log P of selected opioids.
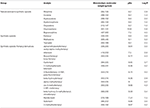
Table 1. Monoisotopic molecular weight (g/mol), pKa and Log P of selected natural, semi-synthetic and constructed opioids.
Volume of distribution (Vd) and protein binding also help to predict the drugs that may exhibit postmortem redistribution. Vd is divers as the volume into which the full corporeality of the drug would have to be uniformly distributed to achieve the concentrations measured in plasma. It is expressed in 50/kg of torso weight (amount of drug in the body divided by the plasma drug concentration). Drugs highly leap to plasma proteins but not to tissue components would be expected to have a pocket-sized Vd, while those drugs which distribute into muscle, adipose tissue and other intracellular components will have a high Vd. Drugs with a Vd greater than 3 L/kg are considered to take a greater potential to undergo postmortem redistribution. Tabular array two summarizes the Vd and poly peptide binding information currently available for selected opioids.
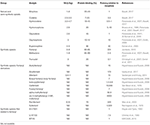
Tabular array 2. Critical pharmacological backdrop in postmortem toxicology, volume of distributon (Vd), protein bining and potency relative to morphine, of selected natural, semi-constructed and synthetic opioids.
1 of the disquisitional bug related to fentanyl, its derivatives and the new synthetic opioids, is the low concentrations expected in the biological samples (ng to pg/mL or ng to pg/one thousand range) due to their high potency. Still, the dominance of these type of drugs varies considerably within this group, and therefore the concentrations reported show a broad range, depending on the drug. Table 2 summarizes the potencies relative to morphine for selected opioids.
Metabolism
The identification and quantification of metabolites in postmortem samples may better the estimation of the analytical results. The determination of metabolites may extend the window of detection, and besides can be employed to calculate metabolite-to-parent ratios in urine and other biological samples to differentiate acute or delayed death. In certain cases, as it happens in morphine and buprenorphine, metabolites can exist pharmacologically active. Although this type of information is limited in the case of the constructed opioids, fentanyl, sufentanil, and alfentanil'south metabolites are inactive in the opioid system (Schneider and Brune, 1986).
Although the utility of metabolite determination in biological samples is known, its awarding to authentic specimens is still scarce in the case of synthetic opioids due to the limited data available about their metabolism (Poklis et al., 2015; Staeheli et al., 2022; Martucci et al., 2017; Allibe et al., 2018). Recent publications almost the identification of new metabolites of the synthetic opioids in vivo and in vitro are available (Wohlfarth et al., 2022; Steuer et al., 2017; Watanabe et al., 2017; Krotulski et al., 2018a). While in vitro studies utilizing human being liver hepatocytes or microsomes can identify multiple primary and secondary metabolites for a particular fentanyl derivative, actual man specimens typically show lower number and/or a different metabolite prevalence profile, so studies investigating the presence of the in vitro metabolites in authentic homo samples are highly encouraged. Table three summarizes contempo publications nigh the identification of new metabolites of synthetic opioids in vitro and in vivo.
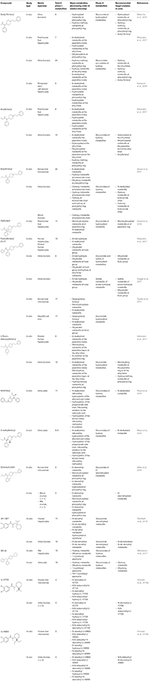
Table three. In vitro and in vivo metabolism of synthetic opioids.
Fentanyl-derivatives metabolism studies showed similarities and differences from fentanyl metabolism pathways and rates. These unlike metabolic pathways observed for sure derivatives, demonstrate the need to perform private metabolism studies for each new compound. In the example of fentanyl, only less than 8% of fentanyl is excreted unchanged. Approximately 85% of the dose is excreted inside 72 h in feces and urine, the majority as metabolites mainly equally norfentanyl generated by N-dealkylation at the piperidine nitrogen (McClain and Hug, 1980). Minor fentanyl metabolites are despropionylfentanyl, likewise known as 4-ANPP, which is formed past carboxamide hydrolysis, and hydroxyfentanyl and hydroxynorfentanyl metabolites, both hydroxylated at the propionyl moiety (Goromaru et al., 1984; Mahlke et al., 2014).
Several synthetic opioids follow a similar metabolic pathway to fentanyl. Alfentanil undergoes piperidine Due north-dealkylation to noralfentanil (Meuldermans et al., 1988). Major alpha-methylfentanyl metabolites in rats were norfentanyl and hydroxypropionyl norfentanyl metabolites, exactly as fentanyl (Sato et al., 2010). Meyer et al. (2012) investigated the metabolism in rats of isofentanyl and 3-methyl fentanyl. After the assistants of suspected recreational doses, the parent drugs could non be detected in urine and their common nor-metabolite was the predominant compound.
Patton et al. (2014) detected high concentrations of acetylfentanyl and acetyl norfentanyl (>16,500 ng/mL, 180 min mail-dose) in urine samples from rats treated with a toxic dose of acetylfentanyl (3 mg/kg); all the same, Melent'ev et al. (2015), showed that the principal pathway of the biotransformation of acetylfentanyl was hydroxylation by the phenylethyl moiety rather than N-dealkylation in authentic man samples. Melent'ev et al. (2015) and Watanabe et al. (2017) recommended as target analytes in human urine hydroxy-methoxy at phenylethyl moiety and monohydroxylated metabolites, although the reported hydroxylation position in both publications was different. In both publications, the parent compound acetylfentanyl was highly arable in urine samples, indicating that the parent drug is a suitable target.
Acrylfentanyl underwent N-dealkylation at the piperidine nitrogen producing the major nor-metabolite (Watanabe et al., 2017). The parent compound was besides detected at high concentrations in urine samples. N-Dealkylation and monohydroxylation of the piperidine ring were the dominant metabolic pathways for carfentanil in vitro (Feasel et al., 2022). In that study, the authors observed a slow parent depletion in the hepatocytes. For 4-fluoroisobutyrylfentanyl the master metabolites identified in urine were the nor-metabolite, and monohydroxy metabolites at the piperidine ring or at the ethyl linker, equally well every bit the parent compound. In terms of specificity, Watanabe et al., recommended as target compounds in urine the monohydroxy metabolites and the hydroxymethoxy metabolite (Watanabe et al., 2017).
In the case of butyrfentanyl, hydroxylation of the butanamide side chain followed by subsequent oxidation to the carboxylic acid represented the major metabolic step (Steuer et al., 2017). Although the norbutyrfentanyl was not among the most abundant metabolites in man samples in that report, the authors suggested its inclusion every bit a recommended target analyte because it showed a high intensity in the in vitro experiment. In authentic postmortem blood and urine samples, butyrfentanyl was still detected at 66 and 1,000 ng/mL, respectively.
Furanylfentanyl contains a furan group that affects its metabolic contour. This structure seemed to favor the amide hydrolysis, which is the main metabolite in vitro and in vivo (Watanabe et al., 2017). In terms of specificity of the target metabolites, Watanabe et al. (2017) recommended the dihydrodiol-metabolite and Goggin et al. (2017) recommended the same metabolite, besides as the sulfate of the metabolite that results from the amide hydrolysis. As it happened with butyrfentanyl (Steuer et al., 2017), the hepatocyte experiment also suggested loftier prevalence for the nor-metabolite, which was non significantly present in the accurate urine samples, illustrating the need to analyze homo specimens. Furanylfentanyl parent compound was detected in authentic urine samples. For ocfentanyl, the predominant metabolite detected in claret, along with the parent drug, was the O-desmethylated metabolite (Allibe et al., 2018).
In the case of the new synthetic opioids not structurally related to fentanyl, different metabolic pathways has been reported. For AH-7921, the preferred metabolic sites were the amine function and the cyclohexyl ring. The two most dominant metabolites after hepatocyte incubation (likewise identified in a urine case specimen) were desmethyl and di-desmethyl AH-7921. Together with the glucuronidated metabolites, they were recommended as suitable analytical targets for documenting AH-7921 intake (Wohlfarth et al., 2022). In the case of MT-45, Montesano et al reported hydroxy-MT-45-glucuronide and di-hydroxy-MT-45-glucuronide every bit the about arable metabolites in rat urine, while the parent drug was found at concentrations <10 ng/mL after 300 min (Montesano et al., 2017). Although similar in chemic construction, U-47700 and U-49900 showed specific metabolites. N-Desmethyl-U-47700 was identified as the major metabolite in human urine specimens, and N,N-Didesethyl-N-desmethyl-U-49900 was identified equally the virtually abundant metabolite nowadays. Unlike U-47700 specimens, U-49900 was detected in low affluence in urine samples (Krotulski et al., 2018a).
Equally indicated by Watanabe et al. (2017), the target metabolites should more often than not be abundant, specific of the parent drug, and prevalent in most, if non all, case samples. Given the strong structural similarities among emerging designer fentanyls, many of them are coincidentally biotransformed to the exact aforementioned metabolite. This fact can make identification of the specific parent drug in a instance hard. The ability to place minor metabolites that are unique and specific to the parent drug is therefore of considerable importance. 4-ANPP tin be formed past fentanyl and other different fentanyl analogs metabolism, and it is as well a precursor contaminant plant in seized illicit fentanyl and analogs, then its presence is not particularly diagnostic. Other common metabolites are: acetylnorfentanyl from acetyl-alpha-methylfentanyl or acetylfentanyl (Watanabe et al., 2017); norfentanyl from fentanyl, beta-hydroxythiofentanyl and alpha-methyl-fentanyl (Sato et al., 2010); norcarfentanil from carfentanil, sufentanil and remifentanil (Feasel et al., 2022). three,4-dichloro-N-(two-aminocyclohexyl)-N-methyl-benzamide is a common metabolite of U-47700 and U-49900, but it is not a major metabolite in urine for either compound (Krotulski et al., 2018a).
Another of import attribute of the metabolism is the identification of the enzymes involved. Pharmacokinetic interactions may be produced due to the presence of other substances metabolized past the same enzymes, ultimately affecting the drug blood concentrations. Fentanyl, sufentanyl and alfentanil are mainly metabolized by CYP 3A4 (Feierman and Lasker, 1996; Guitton et al., 1997). Steuer et al., identified CYP 3A4 and CYP 2D6 every bit the isoforms involved in the metabolism of butyrfentanyl (Steuer et al., 2017). Meyer et al., reported that CYP 3A4, CYP 3A5 and CYP 2C19 are involved in the metabolism of 3-methylfentanyl and isofentanyl and, in the case of isofentanyl, additionally CYP2D6 (Meyer et al., 2012). Remifentanil is the simply family member of this class establish to be ~95% metabolized in the blood and tissues by non-CYP enzymes, probably due to an easily accessible ester group assuasive rapid hydrolysis by circulating blood esterases (Bürkle et al., 1996).
Concentrations in Postmortem Specimens and Other Findings
The concentrations determined in postmortem specimens varied considerably depending on the type of constructed opioid detected. Derivatives with potencies relative to morphine of more than 170, showed concentrations in femoral blood in the low ng/mL or pg/mL range, while those derivatives with potencies similar to morphine showed concentrations of hundreds, and fifty-fifty thousands, of ng/mL. An exception happens with furanyl fentanyl, which is seven times more potent than morphine (Higashikawa and Suzuki, 2008), but the reported femoral concentrations were less than 50 ng/mL. Typical morphine postmortem concentrations in blood in fatalities are from 200 to ii,300 ng/mL, for methadone 400 to one,800 ng/mL, for buprenorphine 1.i–29 ng/mL and norbuprenorphine (active metabolite) 0.2–13 ng/mL (Baselt, 2017), and for oxymorphone 23–554 ng/mL (Crum et al., 2013). The potency of the unlike drugs affects their lethal levels, merely other important issues, such as the presence of other CNS depressant drugs, and developed opioids tolerance, have to be taken into account in the estimation of the concentrations. The derivative with the highest number of published cases was acrylfetanyl, and with the lowest MT-45. Table 4 summarizes the concentrations of the parent drugs plant in case reports and articles where overdose due to a specific opioid was the cause of death.
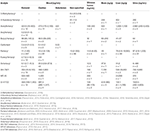
Table 4. Postmortem concentrations in different biological samples for constructed opioids (median, range, number of cases).
In several cases, multiple constructed opioids were detected. Acetylfentanyl and fentanyl were often found together (Pearson et al., 2015; Poklis et al., 2015; Dwyer et al., 2018). Other combinations were butyryl fentanyl and acetyl fentanyl (McIntyre et al., 2022b; Poklis et al., 2022), or U-47700 (Mohr et al., 2022); furanyl fentanyl and acetyl fentanyl (Papsun et al., 2017), acryl fentanyl (Butler et al., 2017), butyryl fentanyl (Mohr et al., 2022), fentanyl (Guerrieri et al., 2017a), or carfentanil (Shanks and Behonick, 2017); carfentanil and fentanyl (Shanks and Behonick, 2017); and tetrahydrofuran fentanyl and U-49900 (Krotulski et al., 2018b). The femoral concentrations reported in those combination cases were oftentimes below the range of the concentrations summarized in Table iv. Acetylfentanyl median and concentration range in multiple constructed opioids cases were 9.4, 0.4–240 ng/mL (northward = 15); acrylfentanyl 0.iii ng/mL (n = 1); butyrfentanyl 14.nine, 0.3–58 ng/mL (n = 4); carfentanil 0.08, 0.05–0.1 ng/mL (due north = 2); fentanyl 8.ii, 1.1–38 ng/mL (n = 14); furanyl fentanyl 1.7, 0.6–6.i ng/mL (n = four) and U-47700 17 ng/mL (n = i).
In all of the reports mentioned in Table iv and higher up, constructed opioids were commonly detected with other drugs, particularly other CNS depressants, such as benzodiazepines, ethanol and other opioids. This combination may produce a pharmacodynamic interactions and increase the risk of respiratory depression. This possible interaction between opioids, alcohol and benzodiazepines has been previously described for other opioids, such as buprenorphine (Häkkinen et al., 2012; Seldén et al., 2012), methadone (Jones et al., 2012; Pilgrim et al., 2013; Nielsen et al., 2015), oxycodone (Ogle et al., 2012), and heroin (Thaulow et al., 2014). Among the reviewed cases positive for constructed opioids other than fentanyl, 44 reported equally cause of death intoxication due to multiple drugs and 77 intoxication mainly due to one specific opioid. The way of death was predominantly accidental (northward = 99), and suicides were reported in vii cases.
Postmortem Redistribution and Stability
Postmortem changes in drug concentrations can happen via postmortem redistribution (PMR) from tissues of a college to a lower concentration. Physicochemical and pharmacological backdrop of the analytes, such as pKa, log P, volume of distribution (Vd) and poly peptide bounden, may signal drugs that experience this postmortem phenomenon. Lipophilic basic drugs with a Vd > 3 L/kg, such equally fentanyl, may undergo PMR. Fentanyl has been reported to undergo extensive PMR (Luckenbill et al., 2008; Olson et al., 2010; Palamalai et al., 2013; Brockbals et al., 2018). In the case of the constructed opioids, limited data is currently bachelor about PMR, and as well as information almost pKa, log P and Vd (Tables 2, 3). Staeheli et al. (2016) reported postmortem concentration changes of butyrfentanyl and metabolites, suggesting these compounds were prone to PMR. PMR reports about other synthetic opioids are not currently available.
Based on currently published instance reports and articles, the cardiac blood-to-femoral claret and liver-to-femoral blood ratios were calculated to predict candidates of PMR. Results are summarized in Table 5. Due to the deficient amount of data available (one–iv cases per analyte), no conclusions could be drawn. Synthetic opioids showed median cardiac-to-femoral ratios around 1, and a trend to accumulate in the liver. Regarding the distribution to vitreous humor, it may exist slow showing higher concentrations in blood. Other factors, such as time of expiry and sample collection, or rapid vs. delayed deaths, has not been taken into account in this assay due to the limited information available.

Table 5. Postmortem concentration ratios in unlike biological samples for synthetic opioids (median, range, number of cases).
PMR is still a controversial issue for archetype opioids. Hargrove and Molina (2014) showed insignificant redistribution of morphine from primal sites within 24 h after death in bodies kept at iv°C, while Staeheli et al. (2017) observed a significant increase of morphine concentration, although these changes were not relevant for forensic interpretation. Morphine-derivatives, such u.s. hydrocodone (Saitman et al., 2015), codeine (Frost et al., 2022), and oxycodone (Brockbals et al., 2018), are unlikely to undergo substantial PMR changes. More lipophilic opioids with higher Vd, similar methadone (Jantos and Skopp, 2013; Holm and Linnet, 2015; Brockbals et al., 2018), may undergo PMR.
Several studies have been conducted to evaluate stability of fentanyl and some of its derivatives in fortified biological samples, such as claret, plasma and urine. Eleven fentanils (fentanyl, norfentanyl, carfentanil, norcarfentanil, sufentanil, norsufentanil, lofentanil, 3-methylfentanyl, alfa-methylfentanyl, ohmefentanyl, and remifentanil acid metabolite), were stable in urine samples stored at −20°C or below for at least 2 months. However, remifentanil in urine samples decreased by approximately ninety% within 1 week at room temperature and by more than l% in samples stored for 1 week at 4°C. Considering of the instability of that analyte, the authors recommended to analyze the chief metabolite, remifentanil acrid (Wang and Bernert, 2006). Fentanyl and its metabolites norfentanyl, despropionylfentanyl and hydroxynorfentanyl were stable in urine after 3 freeze-thaw cycles, and subsequently storage at −20°C for 2 months (Mahlke et al., 2014).
Fentanyl, norfentanyl, acetyl fentanyl and acetyl norfentanyl spiked into whole blood were stable after iii freeze-thaw cycles and at room temperature for 72 h (Poklis et al., 2015). No loss of fentanyl concentration could be observed afterward 3 months of storage at 4–8°C and −20°C in claret samples at 5 and 10 ng/mL (Andresen et al., 2012). However, some other study showed fentanyl and its metabolites norfentanyl, despropionylfentanyl and hydroxynorfentanyl lose up to 51.6% afterward 3 freeze-thaw cycles, and fentanyl and despropionylfentanyl up to 34.8% afterwards storage at −20°C for 2 months (Mahlke et al., 2014). Furanylfentanyl showed no significant degradation in blood samples at 5 and 10 ng/mL 48 h room temp and at 4°C 7 days (Guerrieri et al., 2017a) and up to 30 days (Mohr et al., 2022).
Regarding the new constructed opioids not related to fentanyl, U-47700 was stable in blood refrigerated for up to 30 days (Mohr et al., 2022). AH-7921 was institute to be stable for at to the lowest degree 21 days in blood and plasma at room temperature (Soh and Elliot, 2014). In the instance of MT-45, a loss of 50% was observed after 12 months of storage (Papsun et al., 2022). Further studies are necessary to evaluate the stability of the unlike synthetic opioids and metabolites, and in additional biological samples of forensic interest, such as vitreous humor and tissues.
Conclusion
We performed a disquisitional review of the currently available literature to aid in the toxicological interpretation of constructed opioids postmortem cases. Synthetic opioids constitute a heterogenous group of compounds related or not to fentanyl, more often than not basic and lipophilic, with a broad range of potencies related to morphine, from 1 to ten,000. Research has been conducted in the investigation of metabolic pathways and identification of target metabolites of fentanyl derivatives and not-structurally related constructed opioids, showing similarities and differences from fentanyl depending on the compound. Postmortem concentrations seemed to correlate with their potency, although the presence of other CNS depressants, such every bit ethanol and benzodiazepines has to exist taken into account. Further enquiry is guaranteed to investigate postmortem redistribution phenomena of this course of compounds, and stability bug in postmortem samples.
Author Contributions
MC and GC contributed conception and pattern of the review. MC, RC, and JP searched, organized, reviewed and analyzed the case reports and enquiry articles. MC wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest Argument
The authors declare that the research was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential conflict of interest.
References
Allibe, Due north., Richeval, C., Phanithavong, M., Faure, A., Allorge, D., Paysant, F., et al. (2018). Fatality involving ocfentanil documented by identification of metabolites. Drug Test. Anal. 10, 995–1000. doi: 10.1002/dta.2326
PubMed Abstract | CrossRef Full Text | Google Scholar
Anderson, D. T., and Muto, J. J. (2000). Duragesic transdermal patch: postmortem tissue distribution of fentanyl in 25 cases. J. Anal. Toxicol. 24, 627–634. doi: x.1093/jat/24.vii.627
PubMed Abstruse | CrossRef Full Text | Google Scholar
Andresen, H., Gullans, A., Veselinovic, K., Anders, S., Schmoldt, A., Iwersen-Bergmann, Due south., et al. (2012). Fentanyl: toxic or therapeutic? postmortem and antemortem blood concentrations after transdermal fentanyl application. J. Anal. Toxicol. 36, 182–194. doi: x.1093/jat/bks005
PubMed Abstract | CrossRef Total Text | Google Scholar
Armenian, P., Vo, Thou. T., Barr-Walker, J., and Lynch, K. L. (2018). Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology 134, 121–132. doi: ten.1016/j.neuropharm.2017.ten.016
PubMed Abstract | CrossRef Full Text | Google Scholar
Baselt, R. C. (2017). Disposition of Toxic Drugs and Chemicals in Man, 11th Edn. Seal Beach, CA: Biomedical Pubns.
Biedrzycki, O. J., Bevan, D., and Lucas, Southward. (2009). Fatal overdose due to prescription fentanyl patches in a patient with sickle prison cell/β-thalassemia and acute chest syndrome: a case report and review of the literature. Am. J. Forensic Med. Pathol. 30, 188–190. doi: 10.1097/PAF.0b013e318187de71
PubMed Abstract | CrossRef Total Text | Google Scholar
Brittain, R. T., Jack, D., and Tyers, M. B. (1977). Pharmacological and sure chemic properties of AH 10407, an unusually short-acting, competitive neuromuscular blocking drug, and some related compounds. Br. J. Pharmacol. 61, 47–55.
PubMed Abstract | Google Scholar
Brockbals, L., Staeheli, S. N., Gascho, D., Ebert, L. C., Kraemer, T., and Steuer, A. E. (2018). Time-dependent postmortem redistribution of opioids in claret and alternative matrices. J. Anal. Toxicol. 42, 365–374. doi: 10.1093/jat/bky017
PubMed Abstract | CrossRef Full Text | Google Scholar
Bruera, Due east., Pereira, J., Watanabe, South., Belzile, M., Kuehn, North., and Hanson, J. (1996). Opioid rotation in patients with cancer hurting. Cancer 78, 852–857. doi: 10.1002/(SICI)1097-0142(19960815)78:four<852::Aid-CNCR23>3.0.CO;2-T
PubMed Abstract | CrossRef Total Text | Google Scholar
Butler, D. C., Shanks, K., Behonick, One thousand. South., Smith, D., Presnell, Due south. E., and Tormos, L. M. (2017). Three cases of fatal acrylfentanyl toxicity in the united states and a review of literature. J. Anal. Toxicol. 42, e6–e11. doi: 10.1093/jat/bkx083
PubMed Abstract | CrossRef Total Text | Google Scholar
Carson, H. J., Knight, L. D., Dudley, M. H., and Garg, U. (2010). A fatality involving an unusual route of fentanyl delivery: chewing and aspirating the transdermal patch. Leg. Med. 12, 157–159. doi: 10.1016/j.legalmed.2010.03.001
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cheney, B. Five., Szmuszkovicz, J., Lahti, R. A., and Zichi, D. A. (1985). Factors affecting binding of trans-North-[ii-(Methylamino)cyclohexyl]benzamides at the primary morphine receptor. J. Med. Chem. 28, 1853–1864. doi: 10.1021/jm00150a017
PubMed Abstruse | CrossRef Full Text | Google Scholar
Christoph, T., Kögel, B., Strassburger, W., and Schug, Due south. A. (2007). Tramadol has a amend potency ratio relative to morphine in neuropathic than in nociceptive pain models. Drugs R D 8, 51–57. doi: ten.2165/00126839-200708010-00005
PubMed Abstruse | CrossRef Full Text | Google Scholar
Coopman, V., and Cordonnier, J. (2017). 'Spice-similar' herbal incense laced with the synthetic opioid U-47700. Toxicol. Anal. Clin. 30, 75–79. doi: 10.1016/j.toxac.2017.07.004
CrossRef Total Text | Google Scholar
Coopman, V., Cordonnier, J., De Leeuw, M., and Cirimele, V. (2016). Ocfentanil overdose fatality in the recreational drug scene. Forensic Sci. Int. 266, 469–473. doi: 10.1016/j.forsciint.2016.07.005
PubMed Abstract | CrossRef Total Text | Google Scholar
Coopman, 5., Cordonnier, J., Pien, K., and Van Varenbergh, D. (2007). LC-MS/MS assay of fentanyl and norfentanyl in a fatality due to awarding of multiple durogesic® transdermal therapeutic systems. Forensic Sci. Int. 169, 223–27. doi: ten.1016/j.forsciint.2006.03.018
CrossRef Total Text | Google Scholar
Crum, E. D., Bailey, Grand. M., Richards-Waugh, L. Fifty., Clay, D. J., Gebhardt, One thousand. A., and Kraner, J. C. (2013). Validation of claret and liver oxymorphone analysis using LC-MS-MS: concentrations in thirty fatal overdoses. J. Anal. Toxicol. 37, 512–516. doi: 10.1093/jat/bkt077
PubMed Abstract | CrossRef Total Text | Google Scholar
Dahan, A., Yassen, A., Bijl, H., Romberg, R., Sarton, E., Teppema, L., et al. (2005). Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br. J. Anaesth. 94, 825–34. doi: 10.1093/bja/aei145
PubMed Abstract | CrossRef Total Text | Google Scholar
Drug Enforcement Administration Department of Justice. (2018). Schedules of Controlled substances: temporary placement of fentanyl related substances in schedule I. Fed. Reg. 83, 5188–5192.
Drummer, O. (2018). Fatalities caused by novel opioids: a review. Forensic Sci. Res. doi: x.1080/20961790.2018.1460063. [Epub ahead of print].
CrossRef Full Text | Google Scholar
Dussy, F. E., Hangartner, S., Hamberg, C., Berchtold, C., Scherer, U., Schlotterbeck, G., et al. (2016). An acute ocfentanil fatality: a case report with postmortem concentrations. J. Anal. Toxicol. 40, 761–766. doi: ten.1093/jat/bkw096
PubMed Abstract | CrossRef Full Text | Google Scholar
Dwyer, J. B., Janssen, J., Luckasevic, T. One thousand., and Williams, K. E. (2018). Study of increasing overdose deaths that include acetyl fentanyl in multiple counties of the southwestern region of the commonwealth of Pennsylvania in 2015–2016. J. Forensic Sci. 63, 195–200. doi: ten.1111/1556-4029.13517
PubMed Abstract | CrossRef Total Text | Google Scholar
Dziadosz, 1000., Klintschar, M., and Teske, J. (2017). Postmortem concentration distribution in fatal cases involving the synthetic opioid U-47700. Int. J. Legal Med. 131, 1555–1556. doi: 10.1007/s00414-017-1593-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Elliott, S. P., Brandt, S. D., and Smith, C. (2016). The first reported fatality associated with the synthetic opioid 3,4-Dichloro-N-[2-(Dimethylamino)Cyclohexyl]-Due north-Methylbenzamide (U-47700) and implications for forensic analysis. Drug Test. Anal. 8, 875–879. doi: 10.1002/dta.1984
CrossRef Total Text | Google Scholar
EMCDDA (2015). Written report on the risk cess of MT-45 in the framework of the Council Decision on new psychoactive substances. Luxembourg: Publications Office of the European Union. Bachelor online at: http://world wide web.emcdda.europa.eu/publications/take chances-assessments/mt-45_en
Feasel, M. G., Wohlfarth, A., Nilles, J. 1000., Pang, Southward., Kristovich, R. L., and Huestis, K. A. (2016). Metabolism of carfentanil, an ultra-potent opioid, in man liver microsomes and human hepatocytes past high-resolution mass spectrometry. AAPS J. 18, 1489–1499. doi: 10.1208/s12248-016-9963-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Feierman, D. E., and Lasker, J. M. (1996). Metabolism of fentanyl, a synthetic opioid analgesic, by human being liver microsomes. Role of CYP3A4. Drug Metab. Disp. 24, 932–939.
PubMed Abstract | Google Scholar
Fels, H., Krueger, J., Sachs, H., Musshoff, F., Graw, Chiliad., Roider, G., et al. (2017). Two fatalities associated with synthetic opioids: AH-7921 and MT-45. Forensic Sci. Int. 277, e30–e35. doi: x.1016/j.forsciint.2017.04.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Frost, J., Løkken, T. N., Helland, A., Nordrum, I. S., and Slørdal, 50. (2016). Post-mortem levels and tissue distribution of codeine, codeine-6-glucuronide, norcodeine, morphine and morphine glucuronides in a serial of codeine-related deaths. Forensic Sci. Int. 262, 128–137. doi: ten.1016/j.forsciint.2016.02.051
PubMed Abstract | CrossRef Full Text | Google Scholar
Gaulier, J.-1000., Richeval, C., Gicquel, T., Hugbart, C., Le Dare, B., Allorge, D., et al. (2017). In vitro characterization of nps metabolites produced by human liver microsomes and the hepaRG cell line using liquid chromatography-high resolution mass spectrometry (LC-HRMS) analysis: application to furanyl fentanyl. Curr. Pharm. Biotechnol. 18, 806–814. doi: 10.2174/1389201018666171122124401
CrossRef Full Text | Google Scholar
Goggin, Chiliad. One thousand., Nguyen, A., and Janis, One thousand. C. (2017). Identification of unique metabolites of the designer opioid furanyl fentanyl. J. Anal. Toxicol. 41, 367–375. doi: x.1093/jat/bkx022
PubMed Abstract | CrossRef Full Text | Google Scholar
Goromaru, T., Matsuura, H., Yoshimura, N., Miyawaki, T., Sameshima, T., Miyao, J., et al. (1984). Identification and quantitative determination of fentanyl metabolites in patients past gas chromatography-mass spectrometry. Anesthesiology 61, 73–77. doi: ten.1097/00000542-198407000-00013
PubMed Abstract | CrossRef Full Text | Google Scholar
Guerrieri, D., Rapp, Eastward., Roman, M., Druid, H., and Kronstrand, R. (2017a). Postmortem and toxicological findings in a series of furanylfentanyl-related deaths. J. Anal. Toxicol. 41, 242–249. doi: 10.1093/jat/bkw129
PubMed Abstract | CrossRef Full Text | Google Scholar
Guerrieri, D., Rapp, E., Roman, One thousand., Thelander, G., and Kronstrand, R. (2017b). Acrylfentanyl: some other new psychoactive drug with fatal consequences. Forensic Sci. Int. 277, e21–e29. doi: 10.1016/j.forsciint.2017.05.010
PubMed Abstract | CrossRef Total Text | Google Scholar
Guitton, J., Desage, Grand., Alamercery, S., Dutruch, L., Dautraix, Due south., Perdrix, J. P., et al. (1997). Gas chromatographic – mass spectrometry and gas chromatographic – fourier transform infrared spectroscopy assay for the simultaneous identification of fentanyl metabolites. J. Chromatogr. B 59, 59–70. doi: 10.1016/S0378-4347(97)00050-9
CrossRef Total Text | Google Scholar
Häkkinen, Thou., Launiainen, T., Vuori, East., and Ojanperä, I. (2012). Benzodiazepines and booze are associated with cases of fatal buprenorphine poisoning. Eur. J. Clin. Pharmacol. 68, 301–309. doi: ten.1007/s00228-011-1122-4
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hayes, A. M., and Tyers, M. B. (1983). Determination of receptors that mediate opiate side effects in the mouse. Br. J. Pharmacol. 79, 731–736. doi: 10.1111/j.1476-5381.1983.tb10011.x
PubMed Abstract | CrossRef Total Text | Google Scholar
Higashikawa, Y., and Suzuki, South. (2008). Studies on ane-(2-Phenethyl)-4-(N-Propionylanilino)Piperidine (Fentanyl) and its related compounds. VI. structure-analgesic activeness relationship for fentanyl, methyl-substituted fentanyls and other analogues. Forensic Toxicol. 26, one–v. doi: 10.1007/s11419-007-0039-ane
CrossRef Full Text | Google Scholar
Hikin, L., Smith, P. R., Ringland, E., Hudson, S., and Morley, Due south. R. (2018). Multiple fatalities in the north of england associated with constructed fentanyl analogue exposure: detection and quantitation a instance serial from early 2017. Forensic Sci. Int. 282, 179–183. doi: 10.1016/j.forsciint.2017.11.036
PubMed Abstruse | CrossRef Total Text | Google Scholar
Holm, K. Yard., and Linnet, One thousand. (2015). Distribution of enantiomers of methadone and its chief metabolite eddp in human tissues and blood of postmortem cases. J. Forensic Sci. 60, 95–101. doi: 10.1111/1556-4029.12627
PubMed Abstract | CrossRef Full Text | Google Scholar
Jantos, R., and Skopp, G. (2013). Postmortem claret and tissue concentrations of R- and S-enantiomers of methadone and its metabolite EDDP. Forensic Sci. Int. 226, 254–60. doi: 10.1016/j.forsciint.2013.01.038
PubMed Abstract | CrossRef Full Text | Google Scholar
Jones, A. Due west., Holmgren, A., and Ahlner, J. (2012). Claret methadone concentrations in living and deceased persons: variations over time, field of study demographics, and relevance of coingested drugs. J. Anal. Toxicol. 36, 12–18. doi: x.1093/jat/bkr013
PubMed Abstract | CrossRef Total Text | Google Scholar
Kanamori, T., Togawa Iwata, Y., Segawa, H., Yamamuro, T., Kuwayama, Thou., Tsujikawa, K., et al. (2018). Metabolism of fentanyl and acetylfentanyl in human-induced pluripotent stem cell-derived hepatocytes. Biol. Pharm. Bull. 41, 106–114. doi: ten.1248/bpb.b17-00709
PubMed Abstract | CrossRef Full Text | Google Scholar
Karinen, R., Tuv, S. Southward., Rogde, Southward., Peres, Thou. D., Johansen, U., Frost, J., et al. (2014). Lethal poisonings with AH-7921 in combination with other substances. Forensic Sci. Int. 244, e21–e24. doi: x.1016/j.forsciint.2014.08.013
PubMed Abstract | CrossRef Total Text | Google Scholar
Krinsky, C. S., Lathrop, S. Fifty., Crossey, M., Baker, Thousand., and Zumwalt, R. (2011). A toxicology-based review of fentanyl-related deaths in new mexico (1986–2007). Am. J. Forensic Med. Pathol. 32, 347–351. doi: x.1097/PAF.0b013e31822ad269
PubMed Abstract | CrossRef Full Text | Google Scholar
Krinsky, C. S., Lathrop, Due south. 50., and Zumwalt, R. (2014). An examination of the postmortem redistribution of fentanyl and interlaboratory variability. J. Forensic Sci. 59, 1275–1279. doi: 10.1111/1556-4029.12381
PubMed Abstract | CrossRef Total Text | Google Scholar
Kronstrand, R., Thelander, G., Lindstedt, D., Roman, Grand., and Kugelberg, F. C. (2014). Fatal intoxications associated with the designer opioid AH-7921. J. Anal. Toxicol. 38, 599–604. doi: 10.1093/jat/bku057
PubMed Abstract | CrossRef Full Text | Google Scholar
Krotulski, A. J., Mohr, A. L. A., Papsun, D. Thousand., and Logan, B. Thou. (2018a). Metabolism of novel opioid agonists U-47700 and U-49900 using human being liver microsomes with confirmation in authentic urine specimens from drug users. Drug Test. Anal. x, 127–136. doi: 10.1002/dta.2228
PubMed Abstruse | CrossRef Full Text | Google Scholar
Krotulski, A. J., Papsun, D. M., Friscia, 1000., Swartz, J. L., Holsey, B. D., and Logan, B. K. (2018b). Fatality following ingestion of tetrahydrofuranylfentanyl, U-49900 and Methoxy-Phencyclidine. J. Anal. Toxicol. 42, e27–32. doi: 10.1093/jat/bkx092
PubMed Abstract | CrossRef Full Text | Google Scholar
Kuhlman, J. J., McCaulley, R., Valouch, T. J., and Behonick, G. South. (2003). Fentanyl use, misuse, and corruption: a summary of 23 postmortem cases. J. Anal. Toxicol. 27, 499–504. doi: x.1093/jat/27.7.499
PubMed Abstruse | CrossRef Total Text | Google Scholar
Liu, L., Wheeler, S. Due east., Venkataramanan, R., Rymer, J. A., Pizon, A. F., Lynch, Grand. J., et al. (2018). Newly emerging drugs of corruption and their detection methods. Am. J. Clin. Pathol. 149, 105–116. doi: 10.1093/ajcp/aqx138
PubMed Abstract | CrossRef Full Text | Google Scholar
Luckenbill, Yard., Thompson, J., Middleton, O., Kloss, J., and Apple, F. (2008). Fentanyl postmortem redistribution: preliminary findings regarding the relationship amidst femoral blood and liver and middle tissue concentrations. J. Anal. Toxicol. 32, 639–643. doi: ten.1093/jat/32.8.639
PubMed Abstract | CrossRef Total Text | Google Scholar
Mahlke, N. Southward., Ziesenitz, Five., Mikus, Grand., and Skopp, G. (2014). Quantitative low-volume assay for simultaneous determination of fentanyl, norfentanyl, and modest metabolites in human being plasma and urine past liquid chromatography—tandem mass spectrometry (LC-MS/MS). Int. J. Legal Med. 128, 771–778. doi: 10.1007/s00414-014-1040-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Marchei, Due east., Pacifici, R., Mannocchi, G., Marinelli, Eastward., Busardò, F. P., and Pichini, Southward. (2018). New constructed opioids in biological and non-biological matrices: a review of electric current analytical methods. Trends Anal. Chem. 102, i–fifteen. doi: x.1016/j.trac.2018.01.007
CrossRef Total Text | Google Scholar
Marinetti, L. J., and Ehlers, B. J. (2014). A series of forensic toxicology and drug seizure cases involving illicit fentanyl alone and in combination with heroin, cocaine or heroin and cocaine. J. Anal. Toxicol. 38, 592–598. doi: 10.1093/jat/bku086
CrossRef Full Text | Google Scholar
Martin, T. L., Woodall, K. 50., and McLellan, B. A. (2006). Fentanyl-related deaths in ontario, canada: toxicological findings and circumstances of death in 112 cases (2002-2004). J. Anal. Toxicol. 30, 603–610. doi: 10.1093/jat/30.8.603
PubMed Abstract | CrossRef Full Text | Google Scholar
Martucci, H. F. H., Ingle, Due east. A., Hunter, M. D., and Rodda, 50. N. (2017). Distribution of furanyl fentanyl and iv-ANPP in an accidental acute expiry: a instance report. Forensic Sci. Int. 283, e13–e17. doi: 10.1016/j.forsciint.2017.12.005
PubMed Abstract | CrossRef Full Text | Google Scholar
McIntyre, I. M., Gary, R. D., Estrada, J., and Nelson, C. L. (2014). Antemortem and postmortem fentanyl concentrations: a instance report. Int. J. Legal Med. 128, 65–67. doi: ten.1007/s00414-013-0897-5
PubMed Abstract | CrossRef Full Text
McIntyre, I. M., Trochta, A., Gary, R. D., Malamatos, Yard., and Lucas, J. R. (2016a). An astute acetyl fentanyl fatality: a case study with postmortem concentrations. J. Anal. Toxicol. 40:88. doi: 10.1093/jat/bkv043
CrossRef Total Text
McIntyre, I. M., Trochta, A., Gary, R. D., Wright, J., and Mena, O. (2016b). An acute butyrfentanyl fatality: a example written report with postmortem concentrations. J. Anal. Toxicol. 40, 162–166. doi: ten.1093/jat/bkv138
CrossRef Full Text | Google Scholar
Melent'ev, A. B., Kataev, South. S., and Dvorskaya, O. N. (2015). Identification and analytical properties of acetyl fentanyl metabolites. J. Anal. Chem. 70, 240–248. doi: ten.1134/S1061934815020124
CrossRef Full Text | Google Scholar
Meuldermans, W., Van Peer, A., Hendrickx, J., Woestenborghs, R., Lauwers, Due west., Heykants, J., et al. (1988). Alfentanil pharmacokinetics and metabolism in humans. Anesthesiology 69, 527–534. doi: 10.1097/00000542-198810000-00012
PubMed Abstruse | CrossRef Full Text | Google Scholar
Meyer, G. R., Dinger, J., Schwaninger, A. East., Wissenbach, D. K., Zapp, J., Fritschi, G., et al. (2012). Qualitative studies on the metabolism and the toxicological detection of the fentanyl-derived designer drugs 3-methylfentanyl and isofentanyl in rats using liquid chromatography-linear ion trap-mass spectrometry (LC-MS N). Anal. Bioanal. Chem. 402, 1249–1255. doi: ten.1007/s00216-011-5528-8
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mohr, A. 50., Friscia, M., Papsun, D., Kacinko, S. L., Buzby, D., and Logan, B. One thousand. (2016). Analysis of novel synthetic opioids U-47700, U-50488 and furanyl fentanyl by LC-MS/MS in postmortem casework. J. Anal. Toxicol. twoscore, 709–717. doi: 10.1093/jat/bkw086
PubMed Abstract | CrossRef Full Text | Google Scholar
Montesano, C., Vannutelli, G., Fanti, F., Vincenti, F., Gregori, A., Rita Togna, A., et al. (2017). Identification of MT-45 metabolites: in silico prediction, in vitro incubation with rat hepatocytes and in vivo confirmation. J. Anal. Toxicol. 41, 688–697. doi: 10.1093/jat/bkx058
PubMed Abstract | CrossRef Full Text | Google Scholar
Moore, P. West., Palmer, R. B., and Donovan, J. West. (2015). Fatal fentanyl patch misuse in a hospitalized patient with a postmortem increase in fentanyl blood concentration. J. Forensic Sci. threescore, 243–246. doi: 10.1111/1556-4029.12559
PubMed Abstract | CrossRef Total Text | Google Scholar
Nielsen, M. K., Johansen, S. South., and Linnet, Thousand. (2015). Evaluation of poly-drug apply in methadone-related fatalities using segmental pilus analysis. Forensic Sci. Int. 248, 134–139. doi: x.1016/j.forsciint.2015.01.004
PubMed Abstruse | CrossRef Full Text | Google Scholar
Niemegeers, C. J., Schellekens, K. H., Van Bever, Westward. F., and Janssen, P. A. (1976). Sufentanil, a very strong and extremely safe intravenous morphine-like chemical compound in mice, rats and dogs. Arzneimittelforschung 26, 1551–1556.
PubMed Abstract | Google Scholar
O'Donnell, J. Chiliad., Halpin, J., Mattson, C. L., Goldberger, B. A., and Gladden, R. M. (2017). Deaths Involving Fentanyl, Fentanyl Analogs, and U-47700 — 10 States, July–December 2022. MMWMorbidity R Mortal. Weekly Report 66, 1197–1202.
PubMed Abstract
Oertel, R., Pietsch, J., Arenz, Due north., Zeitz, S. G., Goltz, Fifty., and Kirch, W. (2011). Distribution of metoprolol, tramadol, and midazolam in human being autopsy cloth. J. Chromatogr. A 1218, 4988–94. doi: 10.1016/j.blush.2010.12.113
PubMed Abstract | CrossRef Total Text | Google Scholar
Ogle, A., Moore, K., Barrett, B., Young, M. S., and Pearson, J. (2012). Clinical history and characteristics of persons with oxycodone-related deaths in hillsborough county, Florida in 2009. Forensic Sci. Int. 223, 47–52. doi: 10.1016/j.forsciint.2012.07.016
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ojanperä, I., Gergov, M., Rasanen, I., Lunetta, P., Toivonen, S., Tiainen, E., et al. (2006). Blood levels of 3-methylfentanyl in 3 fatal poisoning cases. American J. Forensic Med. Pathol. 27, 328–331. doi: x.1097/01.paf.0000188097.78132.e5
PubMed Abstract | CrossRef Total Text
Olson, Grand. N., Luckenbill, M., Thompson, J., Middleton, O., Geiselhart, R., Mills, K. Grand., et al. (2010). Postmortem redistribution of fentanyl in blood. Am. J. Clin. Pathol. 133, 447–453. doi: 10.1309/AJCP4X5VHFSOERFT
PubMed Abstract | CrossRef Full Text | Google Scholar
Palamalai, V., Olson, K. N., Kloss, J., Middleton, O., Mills, M., Strobl, A. Q., et al. (2013). Superiority of postmortem liver fentanyl concentrations over peripheral blood influenced by postmortem interval for determination of fentanyl toxicity. Clin. Biochem. 46, 598–602. doi: ten.1016/j.clinbiochem.2013.02.001
PubMed Abstruse | CrossRef Total Text | Google Scholar
Papsun, D., Hawes, A., Mohr, A. 50. A., Friscia, K., and Logan, B. Yard. (2017). Instance series of novel illicit opioid-related deaths. Acad. Forensic Pathol. vii, 477–486. doi: 10.23907/2017.040
CrossRef Total Text | Google Scholar
Papsun, D., Krywanczyk, A., Vose, J. C., Bundock, E. A., and Logan, B. K. (2016). Assay of MT-45, a novel synthetic opioid, in human whole blood by LC-MS-MS and its identification in a drug-related death. J. Anal. Toxicol. xl, 313–317. doi: 10.1093/jat/bkw012
PubMed Abstract | CrossRef Total Text | Google Scholar
Patton, A. L., Seely, Yard. A., Pulla, S., Rusch, North. J., Moran, C. L., Fantegrossi, Due west. E., et al. (2014). Quantitative measurement of acetyl fentanyl and acetyl norfentanyl in human urine by LC-MS/MS. Anal. Chem. 86, 1760–1766. doi: 10.1021/ac4036197
PubMed Abstract | CrossRef Full Text | Google Scholar
Pearson, J., Poklis, J., Poklis, A., Wolf, C., Mainland, One thousand., Pilus, L., et al. (2015). Postmortem toxicology findings of acetyl fentanyl, fentanyl, and morphine in heroin fatalities in tampa, Florida. Acad. Forensic Pathol. 5, 676–689. doi: ten.23907/2015.072
PubMed Abstract | CrossRef Total Text | Google Scholar
Pichini, Due south., Solimini, R., Berretta, P., Pacifici, R., and Busardò, F. P. (2018). Acute intoxications and fatalities from illicit fentanyl and analogues. Ther. Drug Monit. 40, 38–51. doi: x.1097/FTD.0000000000000465
PubMed Abstruse | CrossRef Total Text | Google Scholar
Pilgrim, J. Fifty., McDonough, Grand., and Drummer, O. H. (2013). A review of methadone deaths betwixt 2001 and 2005 in Victoria, Australia. Forensic Sci. Int. 226, 216–22. doi: ten.1016/j.forsciint.2013.01.028
PubMed Abstract | CrossRef Full Text | Google Scholar
Poklis, J., Poklis, A., Wolf, C., Hathaway, C., Arbefeville, E., Chrostowski, 50., et al. (2016). 2 fatal intoxications involving butyryl fentanyl. J. Anal. Toxicol. 40, 703–708. doi: 10.1093/jat/bkw048
PubMed Abstract | CrossRef Full Text | Google Scholar
Poklis, J., Poklis, A., Wolf, C., Mainland, M., Hair, L., Devers, Grand., et al. (2015). Postmortem tissue distribution of acetyl fentanyl, fentanyl and their respective nor-metabolites analyzed by ultrahigh performance liquid chromatography with tandem mass spectrometry. Forensic Sci. Int. 257, 435–441. doi: 10.1016/j.forsciint.2015.10.021
PubMed Abstruse | CrossRef Total Text | Google Scholar
Rodda, L. Northward., Pilgrim, J. L., Di Rago, M., Crump, K., Gerostamoulos, D., and Drummer, O. H. (2017). A cluster of fentanyl-laced heroin deaths in 2015 in Melbourne, Australia. J. Anal. Toxicol. 41, 318–324. doi: 10.1093/jat/bkx013
PubMed Abstract | CrossRef Total Text | Google Scholar
Rohrig, T. P., Miller, South. A., and Baird, T. R. (2017). U-47700: a not so new opioid. J. Anal. Toxicol. 42, e12–e14. doi: x.1093/jat/bkx081
CrossRef Full Text | Google Scholar
Rojkiewicz, Chiliad., Majchrzak, Grand., Celinski, R., Kuś, P., and Sajewicz, K. (2017). Identification and physicochemical characterization of 4-fluorobutyrfentanyl (1-((4-Fluorophenyl)(1-Phenethylpiperidin-4-Yl)Amino)Butan-1-One, four-FBF) in seized materials and post-mortem biological samples. Drug Examination. Anal. nine, 405–414. doi: 10.1002/dta.2135
PubMed Abstract | CrossRef Full Text | Google Scholar
Saitman, A., Fitzgerald, R. Fifty., and McIntyre, I. Yard. (2015). Evaluation and comparing of postmortem hydrocodone concentrations in peripheral blood, central blood and liver specimens: a minimal potential for redistribution. Forensic Sci. Int. 247, 36–40. doi: ten.1016/j.forsciint.2014.eleven.031
PubMed Abstract | CrossRef Total Text | Google Scholar
Sato, S., Suzuki, S., Lee, 10. P., and Sato, Thousand. (2010). Studies on 1-(2-Phenethyl)-4-(N-Propionylanilino)Piperidine (Fentanyl) and related compounds. vii. quantification of α-methylfentanyl metabolites excreted in rat urine. Forensic Sci. Int. 195, 68–72. doi: x.1016/j.forsciint.2009.11.014
PubMed Abstract | CrossRef Full Text | Google Scholar
Schneider, E., and Brune, K. (1986). Opioid activeness and distribution of fentanyl metabolites. Naunyn Schmiedeberg's Arch. Pharmacol. 334, 267–274.
PubMed Abstract | Google Scholar
Scientific Working Group for Forensic Toxicology (2013). Scientific Working Grouping for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 37, 452–474. doi: 10.1093/jat/bkt054
CrossRef Full Text
Seldén, T., Ahlner, J., Druid, H., and Kronstrand, R. (2012). Toxicological and pathological findings in a series of buprenorphine related deaths. possible take a chance factors for fatal result. Forensic Sci. Int. 220, 284–90. doi: ten.1016/j.forsciint.2012.03.016
PubMed Abstract | CrossRef Total Text | Google Scholar
Seth, P., Scholl, L., Rudd, R. A., and Bacon, S. (2018). Overdose deaths involving opioids, cocaine, and psychostimulants — United States, 2015 – 2022. MMWR Morb. Mortal. Wkly. Rep. 67, 349–358 doi: ten.15585/mmwr.mm6712a1
PubMed Abstruse | CrossRef Full Text | Google Scholar
Shanks, K. G., and Behonick, Yard. Southward. (2017). Detection of carfentanil by LC-MS-MS and reports of associated fatalities in the The states. J. Anal. Toxicol. 41, 466–472. doi: 10.1093/jat/bkx042
PubMed Abstract | CrossRef Full Text | Google Scholar
Smith, H. Southward. (2009). Clinical pharmacology of oxymorphone. Pain Med. 10(Suppl. 1), S1–S10. doi: 10.1111/j.1526-4637.2009.00594.x
CrossRef Total Text | Google Scholar
Staeheli, S. Due north., Baumgartner, G. R., Gauthier, S., Gascho, D., Jarmer, J., Kraemer, T., et al. (2016). Time-dependent postmortem redistribution of butyrfentanyl and its metabolites in blood and alternative matrices in a case of butyrfentanyl intoxication. Forensic Sci. Int. 266, 170–177. doi: 10.1016/j.forsciint.2016.05.034
PubMed Abstract | CrossRef Full Text | Google Scholar
Staeheli, Due south. N., Gascho, D., Ebert, L. C., Kraemer, T., and Steuer, A. Due east. (2017). Time-dependent postmortem redistribution of morphine and its metabolites in blood and culling matrices—application of ct-guided biopsy sampling. Int. J. Legal Med. 131, 379–389. doi: 10.1007/s00414-016-1485-ii
PubMed Abstract | CrossRef Full Text | Google Scholar
Steuer, A. E., Williner, E., Staeheli, S. N., and Kraemer, T. (2017). Studies on the metabolism of the fentanyl- derived designer drug butyrfentanyl in human in vitro liver preparations and authentic human samples using liquid chromatography-high resolution mass spectrometry (LC-HRMS). Drug Examination. Anal. nine, 1085–1092. doi: 10.1002/dta.2111
PubMed Abstract | CrossRef Full Text | Google Scholar
Swanson, D. K., Hair, L. S., Strauch Rivers, S. R., Smyth, B. C., Brogan, S. C., Ventoso, A. D., et al. (2017). Fatalities involving carfentanil and furanyl fentanyl: two case reports. J. Anal. Toxicol. 41, 498–502. doi: x.1093/jat/bkx037
PubMed Abstract | CrossRef Full Text | Google Scholar
Takase, I., Koizumi, T., Fujimoto, I., Yanai, A., and Fujimiya, T. (2016). An autopsy case of acetyl fentanyl intoxication caused by insufflation of 'Designer Drugs.' Legal Med. 21, 38–44. doi: 10.1016/j.legalmed.2016.05.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Thaulow, C. H., Høiseth, G., Andersen, J. M., Handal, Yard., and Mørland, J. (2014). Pharmacokinetic interactions between ethanol and heroin: a study on mail service-mortem cases. Forensic Sci. Int. 242, 127–134. doi: 10.1016/j.forsciint.2014.06.032
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ujváry, I., Jorge, R., Christie, R., Le Ruez, T., Danielsson, H. V., Kronstrand, R., et al. (2017). Acryloylfentanyl, a recently emerged new psychoactive substance: a comprehensive review. Forensic Toxicol. 35, 232–243. doi: 10.1007/s11419-017-0367-8
CrossRef Full Text | Google Scholar
UNODC (2017). Recommended Methods for the Identification and Assay of Fentanyl and Its Analogues in Biological Specimens (Vienna).
Van Bever, Due west. F., Niemegeers, C. J., Schellekens, K. H., and Janssen, P. A. (1976). Northward-4-Substituted 1-(2-Arylethyl)-4-Piperidinyl-Due north-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneimittelforschung. 26, 1548–1551.
PubMed Abstract | Google Scholar
Vardanyan, R. South., and Hruby, V. J. (2014). Fentanyl-related compounds and derivatives: curresnt condition future prospects for pharmaceutical applications. Future Med. Chem. 6, 385–412. doi: ten.4155/fmc.thirteen.215
PubMed Abstract | CrossRef Full Text | Google Scholar
Vorce, S. P., Knittel, J. L., Holler, J. M., Magluilo, J., Levine, B., Berran, P., et al. (2014). A fatality involving Ah-7921. J. Anal. Toxicol. 38, 226–230. doi: 10.1093/jat/bku011
PubMed Abstract | CrossRef Total Text | Google Scholar
Wang, L., and Bernert, J. T. (2006). Assay of 13 fentanils, including sufentanil and caffentanii, in human being urine past liquid ionization-tandem mass spectrometry *. J. Anal. Toxicol. 30, 335–341.
Google Scholar
Watanabe, South., Vikingsson, S., Roman, Thousand., Greenish, H., Kronstrand, R., and Wohlfarth, A. (2017). In vitro and in vivo metabolite identification studies for the new synthetic opioids acetylfentanyl, acrylfentanyl, furanylfentanyl, and 4-fluoro-isobutyrylfentanyl. AAPS J. xix, 1102–1122. doi: 10.1208/s12248-017-0070-z
PubMed Abstract | CrossRef Total Text | Google Scholar
Wohlfarth, A., Scheidweiler, K. B., Pang, S., Zhu, Yard., Castaneto, Chiliad., Kronstrand, R., et al. (2016). Metabolic characterization of AH-7921, a synthetic opioid designer drug: in vitro metabolic stability assessment and metabolite identification, evaluation of in silico prediction, and in vivo confirmation. Drug Test. Anal. 8, 779–791. doi: ten.1002/dta.1856
PubMed Abstruse | CrossRef Full Text | Google Scholar
Yonemitsu, One thousand., Sasao, A., Mishima, S., Ohtsu, Y., and Nishitani, Y. (2016). A fatal poisoning case by intravenous injection of 'Bathroom Salts' containing acetyl fentanyl and 4-methoxy PV8. Forensic Sci. Int. 267, e6–e9. doi: 10.1016/j.forsciint.2016.08.025
PubMed Abstract | CrossRef Total Text | Google Scholar
castillothely1938.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fphar.2018.01210/full
0 Response to "How Can I Get a Lethal Dose of Fentanyl"
Post a Comment